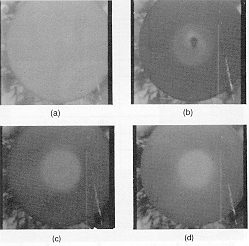
 This figure shows four different frames captured from the IR video camera
at different times for a run using a relatively volatile solvent (acetone)
on a glass substrate. As an IR image, the brightness indicates the relative
temperature (brighter is warmer and darker is cooler). Temperature/colors
are calibrated by using standard black-body emitters. Frame (a) shows the
wafer before fluid of the same temperature is dispensed. Frames (b), (c),
and (d) show time intervals at 1, 3, and 5 seconds into spinning, respectively.
Spin speed was 500 RPM.
This figure shows four different frames captured from the IR video camera
at different times for a run using a relatively volatile solvent (acetone)
on a glass substrate. As an IR image, the brightness indicates the relative
temperature (brighter is warmer and darker is cooler). Temperature/colors
are calibrated by using standard black-body emitters. Frame (a) shows the
wafer before fluid of the same temperature is dispensed. Frames (b), (c),
and (d) show time intervals at 1, 3, and 5 seconds into spinning, respectively.
Spin speed was 500 RPM.
Most of the observed cooling has occurred already within the first second. Then with increased spinning time, the forced air currents gradually help the wafer warm up to the temperature of the surrounding air. With a volatile solvent (as used for this experiment) the fluid residence time on the wafer surface is quite short. The center of the 4" substrate is substantially warmer than the surrounding ring. This is caused by intimate physical contact between the small metal vacuum chuck and the back of the substrate. The vacuum chuck was small enough to use with 2" diameter wafers, so lots of wafer was sticking out over the edge and therefore not affected by any contact with metal.